How to Define What's a Lithium Battery
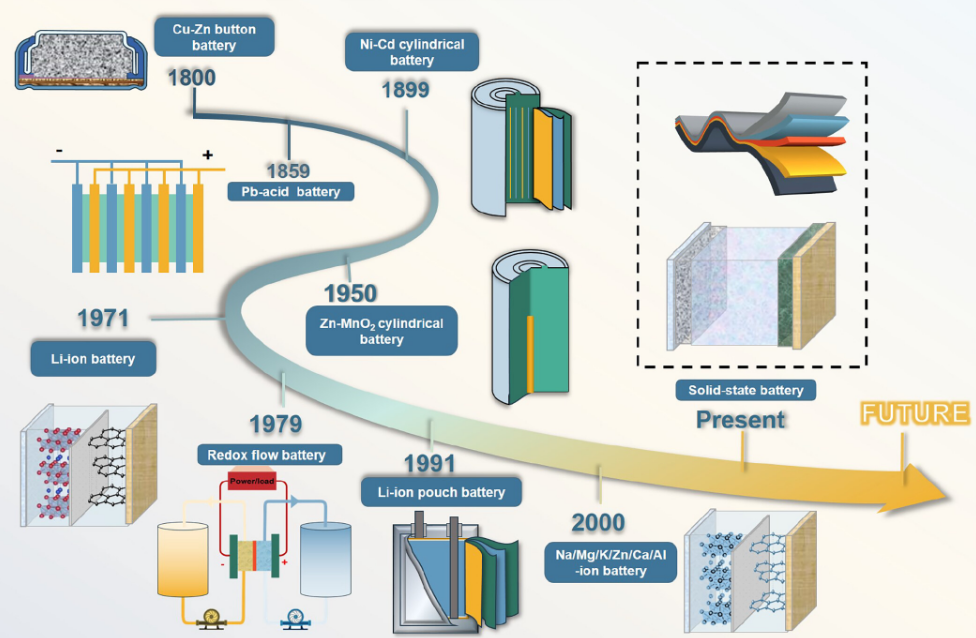
What exactly makes a lithium battery the technology of choice across so many industries? A lithium battery employs lithium ions moving between electrodes to store and release electrical energy through reversible electrochemical reactions. The technology has reshaped entire industries since its commercialization in 1991 and earned its inventors the 2019 Nobel Prize in Chemistry.
The lithium battery industry has become a space where marketing narratives often diverge from manufacturing realities and where billion dollar investment decisions rest on technical misunderstandings that persist because correcting them serves no one's commercial interests.
Understanding Lithium Batteries
A lithium battery functions as a sophisticated electrochemical system engineered to store electrical energy as chemical potential and release it on demand. The technology emerged from decades of research beginning in the 1970s, with M. Stanley Whittingham, John Goodenough, and Akira Yoshino making breakthrough contributions that transformed lithium based concepts into commercially viable rechargeable batteries.
What separates their achievement from incremental battery improvements preceding it was a fundamental reconceptualization. Rather than chemical reactions that physically transform electrode materials, as in lead acid systems where lead sulfate literally grows and dissolves, lithium ion batteries operate through intercalation, a process where ions move between atomic layers of the electrode material without altering its fundamental structure.
Most technical discussions relegate this distinction to historical footnote status, missing its significance as the central insight explaining why lithium batteries function at all. Intercalation permits repeated energy storage and release without the degradation inherent in transformation based chemistry. Every battery chemistry preceding lithium ion suffered from electrodes that physically changed composition during cycling. These changes gradually became irreversible. Intercalation sidesteps this problem entirely. The electrodes remain structurally stable; only the guest ions move.
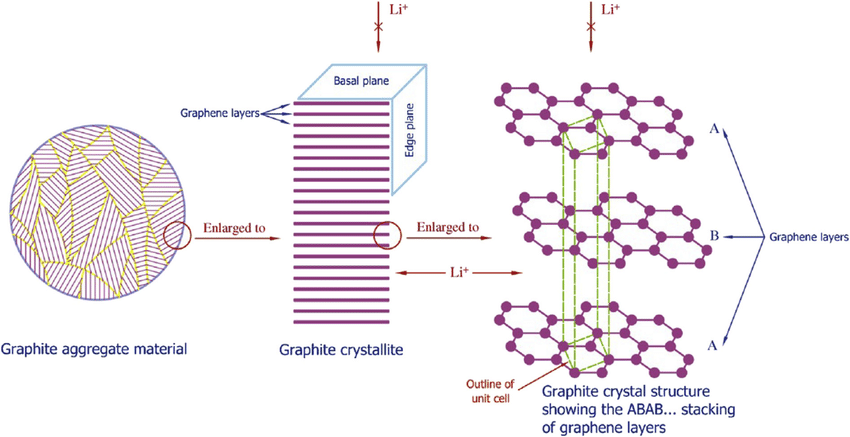
The anode, or negative electrode, typically comprises graphite carbon, serving as the lithium ion storage reservoir during charging. Graphite's layered crystalline structure creates precise gaps between carbon sheets where lithium ions nestle during charge storage.
No engineered material has successfully replicated graphite's combination of low cost, adequate capacity, and structural stability, despite decades of attempts with silicon, lithium metal, and exotic alternatives. Silicon anodes offer theoretically ten times graphite's capacity but swell over 300% during lithiation, mechanically destroying themselves within dozens of cycles. Lithium metal anodes promise the ultimate energy density but grow dendrites that pierce separators and cause fires.
The cathode, or positive electrode, utilizes various metal oxide combinations depending on the specific chemistry employed. Cathode composition serves as the primary differentiator between lithium battery types, determining energy density, safety characteristics, cost structure, and longevity. Marketing departments and technology journalists have fixated on cathode energy density as the paramount metric, treating higher energy density as unambiguous progress. This framing serves companies selling premium high nickel chemistries while obscuring that energy density optimizations often sacrifice safety and longevity in proportions that render the "improvement" net negative for most applications.
Between these electrodes sits a liquid or gel electrolyte that facilitates ion transport, while a porous separator prevents physical contact between electrodes while permitting ionic flow.
The separator, typically a polymer membrane only 20 to 25 micrometers thick, must remain mechanically robust through thousands of cycles while allowing ions to pass freely. When separators fail, batteries fail catastrophically. The Boeing 787 Dreamliner grounding, the Samsung Galaxy Note 7 recall, countless hoverboard fires can all be traced ultimately to separator compromise.
During discharge, lithium ions migrate from the anode through the electrolyte to the cathode, generating a flow of electrons through an external circuit that powers connected devices. When charging, this process reverses as an external power source drives ions back to the anode.
In practice, multiple degradation mechanisms operate simultaneously. Solid electrolyte interphase growth consumes lithium inventory. Electrolyte decomposition generates gases and increases resistance. Cathode particle cracking exposes fresh reactive surfaces. Transition metal dissolution from cathodes poisons anodes.
Cycle life ratings are statistical averages under laboratory conditions that rarely match real world abuse. A battery rated for 2,000 cycles under controlled 25°C testing may deliver only 800 in a vehicle experiencing thermal cycling, vibration, and occasional fast charging.
Lithium's Atomic Advantages
Lithium occupies a unique position in electrochemistry due to its atomic properties. As the third lightest element with the smallest ionic radius among metals, lithium enables exceptionally high voltage and charge storage per unit mass. Energy densities reach 150 to over 300 watt hours per kilogram, three to six times greater than lead acid alternatives.
Lithium sits at the extreme upper left corner of the periodic table, possessing the highest electrochemical potential of any element while carrying minimal atomic mass. No other element offers this combination. Sodium, lithium's nearest competitor, carries roughly three times the atomic mass while delivering lower voltage, relegating sodium ion batteries to stationary applications where weight does not matter regardless of any manufacturing breakthroughs.
The Cathode Chemistry Wars
The term "lithium battery" encompasses at least twelve distinct electrochemical formulations, each optimized for different performance priorities.
Lithium Iron Phosphate

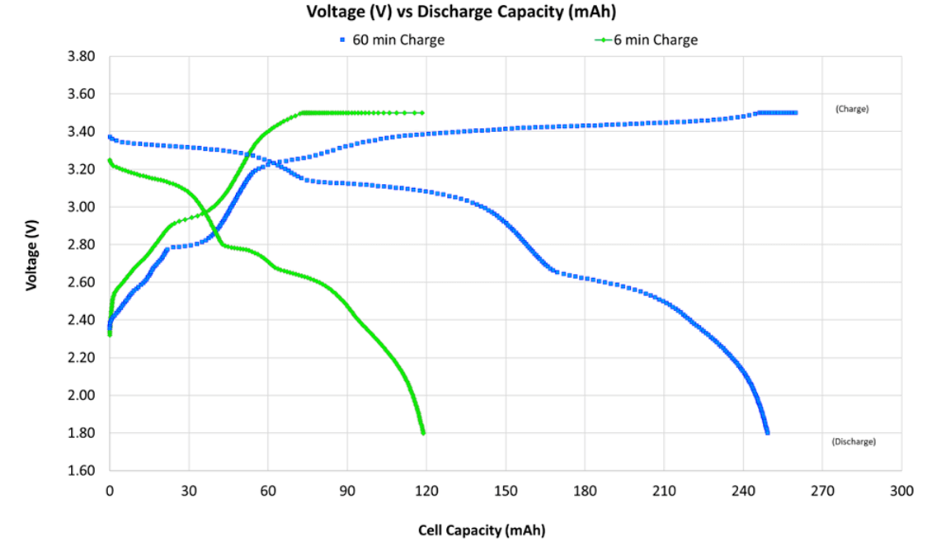
LFP batteries employ phosphate as cathode material and graphite carbon as anode, with nominal voltage of 3.2 volts per cell. The chemistry emerged from 1996 University of Texas research and spent two decades dismissed by Western manufacturers as low energy density compromise technology suitable only for applications where performance mattered less than cost.
LFP's supposed weakness, lower gravimetric energy density compared to nickel rich alternatives, matters only in applications where weight constraints dominate.
For electric vehicles, residential storage, commercial installations, and marine applications, volumetric energy density and cost per kilowatt hour matter more than weight. A vehicle battery pack weighing 450kg versus 400kg changes efficiency marginally; the same battery costing 30% less transforms product economics.
Chinese manufacturers recognized this reality while Western competitors remained focused on laboratory energy density figures. BYD, CATL, and dozens of domestic producers scaled LFP production relentlessly, achieving cost curves that high nickel chemistry mathematically cannot match.
Cobalt trades at $33,000 per ton as of early 2024 according to London Metal Exchange data, with supply concentrated in politically unstable regions. Iron trades below $150 per ton with essentially unlimited supply. No manufacturing efficiency improvement eliminates this input cost differential.
Thermal runaway thresholds sit around 270°C for LFP versus 150 to 200°C for nickel rich chemistries. LFP packs in abuse testing typically smoke and vent rather than achieving sustained combustion.
Cycle life exceeds 3,500 cycles at 80% depth of discharge in independent testing by Argonne National Laboratory, compared to roughly 1,200 for comparable NMC cells. Calendar life also favors LFP because the iron phosphate cathode's extreme stability minimizes parasitic reactions during storage.
LFP batteries now capture over 40% of the global EV market and dominate energy storage deployments. Tesla's decision to shift standard range vehicles to LFP chemistry was perhaps the highest profile acknowledgment of these advantages.

Nickel Rich Chemistries
Lithium Nickel Manganese Cobalt Oxide (NMC) and Lithium Nickel Cobalt Aluminum Oxide (NCA) batteries deliver higher gravimetric energy density through cathode formulations maximizing nickel content while cobalt and manganese or aluminum stabilize the otherwise volatile structure.
This engineering approach reflects an implicit bargain accepting reduced safety margins and longevity in exchange for lighter battery packs enabling longer vehicle range. For premium vehicles where customers pay substantial price premiums for marginal range extensions, this bargain can make economic sense.
The instability of nickel rich cathodes generates consequences across entire product lifecycles.
Higher thermal runaway risk requires more sophisticated thermal management. Liquid cooling systems add cost, weight, and failure modes. Accelerated capacity fade demands larger initial capacity buffers to meet end of life range specifications, partially offsetting theoretical energy density advantages.
Cobalt and nickel price volatility introduces margin uncertainty. Supply chain concentration in potentially hostile nations creates strategic vulnerabilities that governments increasingly recognize as unacceptable.
The chemistry family persists because capital invested in high nickel production lines requires amortization and because marketing departments prefer selling premium products.
Lithium Cobalt Oxide
LCO batteries achieved the highest specific energy among established lithium chemistries but deliver relatively low specific power and cycle life. The chemistry powered early lithium ion commercialization and dominated consumer electronics for two decades.
NMC formulations now approach LCO energy density while delivering superior cycle life and safety. LFP energy density improvements have closed much of the historical gap while reducing costs. LCO's continued presence in product designs reflects engineering inertia rather than technical merit.
The high cobalt content now carries ethical and strategic liabilities. Artisanal cobalt mining in the Democratic Republic of Congo involves documented human rights abuses that sustainability commitments nominally prohibit supporting.
Lithium Titanate
LTO batteries replace standard graphite anodes with lithium titanate. This enables ultra fast charging accepting 10C rates without degradation, cycle life exceeding 15,000 cycles in testing by the National Renewable Energy Laboratory, and operation across extreme temperature ranges from negative 40°C to 60°C.
Energy density drops by roughly 50% compared to graphite anode alternatives.
Applications exist where fast charging capability or extreme cycle life matter more than energy density, including grid frequency regulation, transit buses with opportunity charging, and industrial equipment in harsh environments. LTO batteries serve these applications superbly but remain expensive due to limited production scale.
The Battery Management System
Technical discussions of lithium batteries obsess over cell chemistry while treating battery management systems as implementation details beneath serious attention.
A battery management system monitors cell voltages, temperatures, and current flows while controlling charging, discharging, cell balancing, and safety responses. The BMS determines whether a battery pack achieves its theoretical potential or degrades prematurely due to mismanagement.
Sophisticated BMS implementations can coax 150% of rated cycle life from cells through careful charge management. Crude implementations destroy cells within 50% of specification through overcharge, thermal abuse, and imbalance accumulation.
The quality spread among battery management systems is enormous and largely invisible to purchasers.
Premium automotive BMS implementations monitor individual cell temperatures and voltages, model degradation in real time, adjust charge rates dynamically based on cell condition and thermal state, and balance cells both during charging and operation.
Budget implementations treat battery packs as black boxes, monitoring only pack level metrics while remaining oblivious to cell level variations that compound into failures.
Many batteries blamed on "cell quality" actually died from BMS inadequacy. Temperature sensors too sparse to detect hot spots allowed localized thermal damage to propagate. Charge algorithms optimized for speed rather than longevity accelerated degradation. Passive balancing that only operates during charging allowed imbalances to accumulate in packs that rarely reached full charge.
Thermal Management
Temperature affects lithium battery performance and degradation through mechanisms more consequential than most users appreciate.
A battery operating at 35°C degrades roughly twice as fast as the same battery at 25°C according to studies published in the Journal of Power Sources. This relationship compounds over years of operation into the difference between batteries that last a decade and batteries requiring replacement in three years.
Temperature sensitivity makes thermal management a primary economic driver rather than a mere engineering detail.
A battery installation achieving 20,000 hours of operation at average 30°C temperatures might achieve 35,000 hours at 20°C average, a 75% extension of service life. Investing $50,000 in climate control for a $200,000 battery system becomes economical if it extends replacement intervals by five years.
Thermal management discussions typically focus on safety, specifically preventing thermal runaway during abuse conditions, while ignoring the continuous low level thermal stress that determines calendar and cycle life.
Thermal runaway events, while catastrophic when they occur, remain statistically rare. Thermally accelerated degradation affects every battery in every installation, eroding capacity and increasing resistance in patterns that eventually mandate replacement.
Cold temperature effects deserve equal attention.
Below 0°C, lithium plating risk during charging creates permanent capacity loss and potential safety hazards. Many vehicles in cold climates experience accelerated battery degradation not from cold temperature discharge, which merely reduces available capacity temporarily, but from charging events during or shortly after cold exposure before pack temperatures have equilibrated.
Preheating systems that warm batteries before accepting charge extend cold climate battery longevity. Many system designs omit this protection due to cost pressures.
Solid State Batteries
Solid state batteries replace liquid electrolyte with solid ceramic, glass, or polymer materials. They have attracted billions in investment and media coverage promising revolutionary performance improvements perpetually arriving within five years.
Solid state proponents correctly identify liquid electrolyte as the technology's primary safety limitation. Eliminating flammable organic solvents would enable lithium metal anodes with increased energy density while removing thermal runaway fuel.
The manufacturing challenges have resisted solution for decades.
Solid electrolytes require intimate contact with electrodes across interfaces that must remain stable through thousands of cycles. Achieving this contact during initial manufacturing is possible. Maintaining it as electrodes expand and contract during cycling has proven difficult.
Lithium metal anodes develop interfacial voids during discharge that nucleate failure sites during subsequent charging. Sulfide based solid electrolytes deliver high ionic conductivity but react violently with moisture, requiring manufacturing environments more controlled than conventional lithium ion production.
Commercialization timelines have slipped repeatedly.
Toyota announced solid state battery vehicles for 2020, then 2025, now targeting late this decade. QuantumScape, after going public at a $50 billion valuation in 2020, has yet to ship commercial cells. Some solid state cells now exist in laboratory quantities and limited pilot production, but costs remain multiples of conventional lithium ion with no clear path to parity.
LFP chemistry's safety profile approaches solid state theoretical advantages through different means. Energy density improvements in conventional cells have reduced the gap that solid state promised to close. Fast charging capabilities once imagined as solid state exclusive have been achieved through cell design innovations in liquid electrolyte systems.
Supply Chain Realities
The global lithium battery supply chain has consolidated in China to a degree that generates legitimate strategic concern.
Chinese producers control over three quarters of global cell manufacturing capacity according to BloombergNEF data, with dominance extending upstream into cathode material production, electrolyte manufacturing, and separator production. Mining occurs globally, but refining and processing concentrate overwhelmingly in China.
Western responses, including subsidies for domestic manufacturing and local content requirements, confront structural disadvantages that policy intervention cannot quickly overcome.
Chinese production achieved current scale through decades of sustained investment, workforce development, and supply chain integration. North American and European initiatives starting from lower scale and higher costs face learning curves that extend years regardless of capital availability.
LFP chemistry, increasingly the dominant formulation, requires no cobalt or nickel, eliminating supply chain vulnerabilities that might otherwise justify premium pricing for non Chinese production. The iron phosphate supply chain cannot be weaponized because iron and phosphorus exist abundantly worldwide.
The market appears likely to bifurcate. Premium applications paying for localized supply chains using nickel rich chemistries. Volume applications accepting Chinese LFP dominance as economically inevitable.
Specifications and Selection
Energy Density
Gravimetric energy density in watt hours per kilogram dominates marketing materials despite mattering only for applications with binding weight constraints, primarily aviation and portable consumer electronics.
Ground vehicles face volumetric rather than gravimetric constraints because battery compartments have defined dimensions that accommodate varying weights. Stationary installations care about cost per kilowatt hour and footprint, not weight.
Volumetric energy density in watt hours per liter matters more for most applications but receives less marketing emphasis.
Cycle Life
Cycle life ratings require skeptical evaluation.
Manufacturer specifications typically report cycles to 80% capacity retention under idealized conditions including controlled temperature, moderate C rates, and standardized depth of discharge. Real world operation involves temperature extremes, variable charge rates, and partial cycling at random depths.
Requesting cycle life data under conditions approximating actual application profiles delivers more value than accepting headline specifications. A battery rated for 3,000 cycles at 25°C and 0.3C charge rate might deliver only 1,500 under actual 35°C average temperature and occasional 1C fast charging.
Calendar Life
Batteries degrade whether used or not.
Calendar aging proceeds through parasitic reactions that consume lithium inventory and degrade electrolyte, with rates depending on temperature and state of charge during storage.
A battery stored at full charge at 30°C might lose 20% capacity annually. The same battery at half charge at 20°C might lose only 3% annually.
For applications involving extended storage periods, including backup power, seasonal equipment, and inventory reserves, calendar life dominates cycle life in determining replacement timing.
Round Trip Efficiency
Round trip efficiency, energy output divided by energy input, directly impacts operating economics for applications cycling regularly.
Lithium batteries typically achieve 92% to 98% round trip efficiency depending on chemistry and operating conditions, compared to 75% to 85% for lead acid alternatives.
The efficiency differential compounds across operational life. A solar plus storage system cycling daily at 95% efficiency delivers 10% more usable energy over a decade than an otherwise identical system at 86% efficiency.


Operational Practices
Battery longevity depends heavily on operational practices.
Charge Management
Charging practices offer the greatest leverage for extending battery life. Every charge cycle stresses electrodes through lithium insertion expansion and extraction contraction.
Charging to lower voltage limits extends cycle life. Reducing charge voltage by 100mV per cell, from 4.2V to 4.1V nominal, typically doubles cycle life while sacrificing only 10% to 15% capacity.
Avoiding fast charging except when operationally necessary similarly extends life. Fast charging generates internal heat through resistive losses and concentration polarization. Charging at 0.5C rather than 2C might add 30% to charge duration while reducing degradation rates by similar proportions.
Partial state of charge cycling, maintaining batteries between 20% and 80% rather than cycling to full and empty, reduces stress from both high voltage and low voltage extremes. High voltage promotes electrolyte oxidation. Low voltage promotes copper current collector corrosion.
Temperature Management
Thermal management extends beyond installation climate control to encompass operational practices affecting temperature profiles.
Charging immediately after high power discharge, when batteries remain hot from internal heating, compounds thermal stress. Allowing cooling periods before initiating charging reduces peak temperatures experienced during the charge cycle.
Storage practices significantly impact calendar life. Batteries stored in unconditioned spaces experience temperature cycling that accelerates degradation compared to climate controlled storage.
Cold temperature charging warrants particular caution. Accepting charge current when battery temperatures remain below 0°C risks lithium plating that permanently reduces capacity and creates safety hazards.
Monitoring
Continuous monitoring enables early intervention before minor issues cascade into major failures.
Cell voltage divergence, where individual cells drift from pack average, indicates balancing failures or emerging cell weaknesses. Temperature hot spots suggest localized failures or cooling system degradation. Capacity fade rates exceeding projections indicate operational stresses requiring investigation.
The data for such monitoring typically exists within battery management systems but often goes unexamined until problems manifest as failures.

